Participation Statistics
Virtual Conference Statistics
IPVC 2020 WENT VIRTUAL
IPVC 2020 – 33rd International Papillomavirus Conference & Basic Science, Clinical and Public Health Workshops took place between 20-24 July and for the first time was held as an entirely virtual event.
Overall, the balance between the combination of pre-recorded presentations and live sessions, virtual exhibition and a compact interactive online Virtual Platform allowed a successful dissemination of science and interaction between delegates online at the IPVC20 Virtual Conference.
The IPVC 2020 virtual platform was available from the first day of the Conference, and 3 months after.
Virtual Conference Statistics
The meeting was a great success, close to 1,500 attendees from 90 countries, with over took part in this highly informative meeting.
Virtual Registrations
Countries Represented
Top 20 Countries (%)
- UNITED STATES 29%
- SPAIN 8%
- FRANCE 5%
- UNITED KINGDOM 5%
- CANADA 4%
- NETHERLANDS 3%
- GERMANY 3%
- CHINA 3%
- AUSTRALIA 3%
- BRAZIL 3%
- RUSSIA 2%
- PORTUGAL 2%
- BELGIUM 2%
- SOUTH AFRICA 2%
- MEXICO 2%
- ITALY 2%
- SWEDEN 1%
- SOUTH KOREA 1%
- ARGENTINA 1%
- JAPAN 1%
World Regions (%)
Professional Interest by Field
-
Public Health and Preventive Medicine 29%
-
Infectious Disease 18%
-
Molecular Biology 12%
-
Obstetrics and Gynecology 12%
-
Gynecological Oncology 8%
-
Microbiology 7%
-
Pathology 6%
-
Oncology 5%
-
Allergy and Immunology 2%
-
Pediatrics 1%
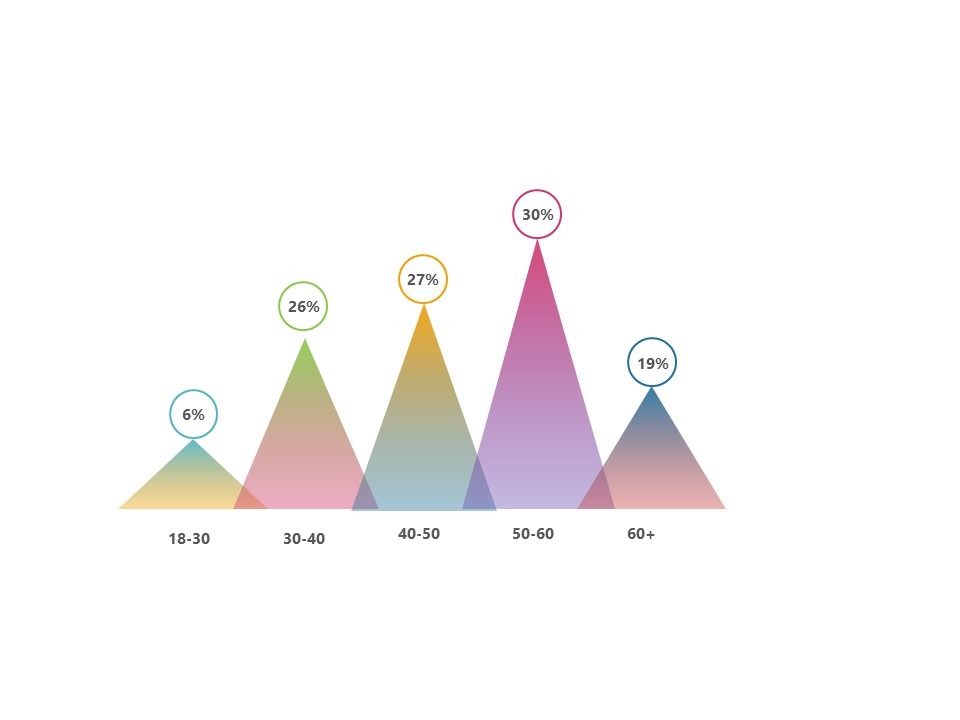
Delegates by Age Group
Delegates by Professional Role
Clinician Researcher
Basic Science Researcher
Clinical Practitioner
Student
Resident/Research Fellow
Industry/Corporate Professional
Nurse/Healthcare Practitioner
Other
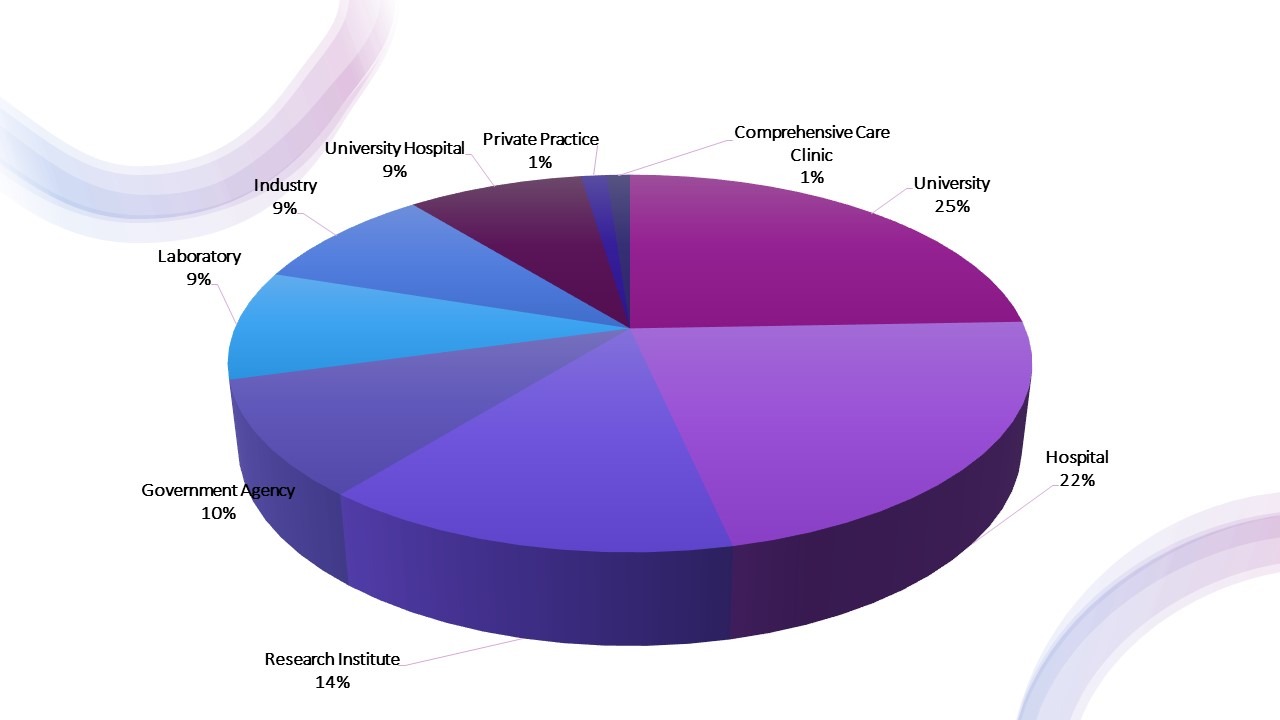
Delegates by Workplace
Commitment to the Highest Standards in CME/CPD
- Championing best practice in CME
- Maintaining and improving standards
- Mentoring and educating
- Working in collaboration with critical stakeholders
CME Accreditation
Industry Supported Sessions
Disclaimer
Information contained in the scientific program must comply with the applicable CME/CPD regulations. Scientific/Educational Program shall not include any commercial elements such as company names, products names, etc. Commercial information shall be kept separate and clearly differentiated from the scientific accredited content. Non educational activities may include promotional elements and shall not be accredited.
Contact us now
for pricing, bookings and customized packages.
